Fat-cell heat production, adipose tissue fatty acids, lipoprotein lipase activity and plasma lipoproteins in adiposis dolorosa
BIRGER FAGHER, MARIO MONTI, PETER NILSSON-EHLE* AND BJORN ÅKESSON*(Received 8 March/5 July 1991; accepted 16 July 1991)
Reprinted from Clinical Science (1991) 81, 793-798SUMMARY
1. Gluteal adipose tissue was examined in 13 patients with generalized adiposis dolorosa, a clinical condition characterized by painful adiposity with a chronic intractable course. The total metabolic activity of fat cells, isolated by collagenase and suspended in Krebs-Ringer bicarbonate buffer with glucose and insulin, was assessed by the measurement of heat production at 37°C using microcalorimetry.
2. Fat cells were markedly enlarged; their metabolic activity expressed in terms of/~W/g, but not in pW/cell, was significantly decreased when compared with both lean and weight-matched non-painful subjects. Both mean values were, however, significantly higher than in grossly obese subjects with similar mean cell size. Heat production as expressed per g of tissue, but not per cell, was inversely correlated with body mass index. One additional patient had unilateral disease, and fat cells from the painful side had a lower heat production than cells from the unaffected side.
3. The fatty acid composition of adipose tissue, as determined by g.c., revealed a significantly increased proportion of monounsaturated (18:1 and 16:1) at the expense of saturated (14:0 and 18:0) fatty acids compared with healthy control subjects. The activity of adipose tissue lipoprotein lipase was slightly, but not significantly, decreased.
4. It is concluded that a metabolic pathogenetic factor cannot be ruled out in adiposis dolorosa. As the results do not explain the nature of the diffuse pain, further studies need to be performed.
Key words: adipose tissue, adiposis dolorosa, calorimetry, fat-cell size, fatty acid composition, heat production, lipids, lipoprotein lipase, obesity, pain.
Correspondence: Dr Birger Fagher, Department of Internal Medicine, University Hospital of Lund, S-221 85 Lund, Sweden.
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LPL, lipopro-tein lipase; TAG, triacylglycerol.
INTRODUCTION
The aetiology of adiposis dolorosa is obscure and so is the cause of the pain and tenderness of the adipose deposits [1-4]. As it is resistant to most analgesics, the disorder may cause great disability. It is therefore important to understand the mechanism behind the remarkable pain and to characterize the tender fat biochemicalty. The present study has focused on the energy metabolism of fat cells in adiposis dolorosa. In order to evaluate a possible metabolic derangement, we employed microcalorimetry to estimate the total metabolic activity of isolated fat cells by measurement of cellular heat production [5]. Studies of fatty acid composition of adipose tissue and lipoprotein lipase (LPL) activity were also performed.
MATERIALS AND METHODS
Patients
Thirteen patients (two males, 11 females) with a clinical diagnosis compatible with adiposis dolorosa were included in the study consecutively. They had the generalized type of disease with a constant moderate to intense pain in their subcutaneous adipose tissue symmetrically over virtually all areas of the body including the face. All were Caucasians. Their mean age was 50 years (range 26-78 years) at the time of biopsy, their body weight was 90 kg (SD 14 kg) and their body mass index (BMI) was 33.5 kg/m2 (SD 5.4 kg/m2). The median duration of pain was 13 years (range 2-48 years) at the time of biopsy. Anthropometric data are detailed in Table 1. One additional patient was examined, a 59-year-old woman, who presented with diffuse fatty accumulations located only at the right buttock and thigh. All subjects were informed of the aim of the study and gave their consent. The Ethical Committee of Lund University gave approval to the invasive procedure.

Pain was dull or burning. All patients reported also excessive weakness, stiffness, disturbed sleep and constant extreme fatigue. Some had even sporadic bouts of low-grade fever without any superimposed infection. An extensive laboratory investigation was performed, and other organic causes of the pain were excluded. Two patients had a history of previous dysthyroidism: patient no. 5, hypothyroidism; patient no. 10, hyperthyroidism. It is noticeable that pain was refractory after they had become euthyroid.
Body weight standards were based on a randomly selected sample of 234 healthy females and 206 males from the city of Lund, after exclusion of subjects with an overweight index of >~ 20% from the original group of 255 and 217 cases, respectively [6]. These standards were normalized for age (21-61 years) and height. The mean height of the 12 female patients, 162 cm (SD 6), did not significantly differ from the standard height of 255 healthy females, 165 cm (SD 6). Total adipose tissue was calculated [7]. Patients were as a rule only mildly obese at the time of onset of the disease, with a mean premorbid body weight of 70 kg (SD 10). Some patients showed rapidly progressive obesity when pain started. Weight reduction efforts, with a transient mean weight loss to 80 kg (SD 13), gave no pain relief; instead, after the 3 years of follow-up they had gained weight to a maximum of 102 kg (SD 19) or a mean BMI of 36.4 kg/m2 (SD 5.3).
D-Thyroxine was previously proposed to diminish the pain in adiposis dolorosa as tested in two cases [8]; eight patients were therefore subjected to 1 month therapy with D-thyroxine (Eulipos, Boehringer Mannhelm), 4 mg once daily, whereafter the above procedure with fat tissue biopsies, etc, was repeated.
Fat-cell heat production
Adipose tissue specimens were obtained by open surgery from the upper lateral gluteal region, where all patients had moderate pain at palpation. It was performed in the morning after half an hour's rest and after an overnight fast. Local anaesthesia with lidocaine was used, without infiltrating the biopsy area. Heat production was measured as described by Monti et al. [5] after isolation of the cells by collagenase treatment [9] of fresh adipose tissue for 20-30 min. With this short treatment no free floating fat was observed in the suspensions, indicating that no significant cell breakage had occurred during preparation. Cells were suspended in Krebs-Ringer bicarbonate buffer (0.05 mol/l; pH 7.4). Glucose (11 mmol/l) and insulin 1100 units/l) were present in the medium in order to provide substrate, thereby stimulating metabolic activity [5]. Mean fat-cell diameter was measured under the light microscope for 100 cells in suspension [9], using a technique that avoided selection of large cells; cellular volume (= p/6 x diameter3) and tri-acylglycerol (TAG) content per cell were calculated from the cell diameter [10]. Duplicate measurements were made in two almost identical microcalorimeters of the thermopile heat conduction type [ 11 ], with the ampoules charged with I ml of cell suspension containing 40% (v/v) cells. Heat production rate refers to the mean of two steady-state values recorded after 1 h at 37°C. The number of cells in each experiment was determined. The coefficient of variation for the method was previously found to be 6.5% [5]. Control biopsies from the subcutaneous gluteal area were obtained by open surgery from 12 lean healthy subjects (seven males, five females) and 15 obese (three males, 12 females) but otherwise healthy subjects, whose values, determined at the same laboratory and with the same equipment, have been reported previously [5, 12]; no sex-related difference was observed. These subjects had mean body weights of 69 kg (SD 8) and 97 kg (SD 14), respectively. In addition, comparison was made with 14 grossly obese subjects [13], weighing 132 kg (SD 27). The age range was 23-45 years in the three control groups (Table 1).
Adipose tissue fatty acid composition, LPL activity and plasma lipoproteins
The fatty acid composition of adipose tissue lipids was measured by g.c. of fatty acid methyl esters, and was compared with that of 25 healthy females [14] with a mean BMI of 26.6 kg/m2 (SD 8.1) and a mean age of 57 years (range 50-70 years). Fat was taken from the upper lateral gluteal area by open surgery in the patients and by a Vacutainer technique [15] in the control subjects, and it was stored at - 70°C. The activity of adipose tissue LPL was measured by a specific assay [16] after elution with heparin [17] and was related to tissue weight. Cholesterol and TAG were measured in plasma by established cnzymic methods; high-density lipoprotein (HDL) cholesterol was determined after precipitation of very-low-density lipoprotein and low-density lipoprotein (LDL) by dextran sulphate and MgCl2, whereafter LDL cholesterol was calculated [18].
Statistics
Means and SDs (standard deviations) are given. Mann-Whitney's U-test was used for inter-group comparisons. Correlation coefficients were calculated by multiple regression analysis (r), or by Spearman's rank test (rs) if values seemed to be not normally distributed.
RESULTS
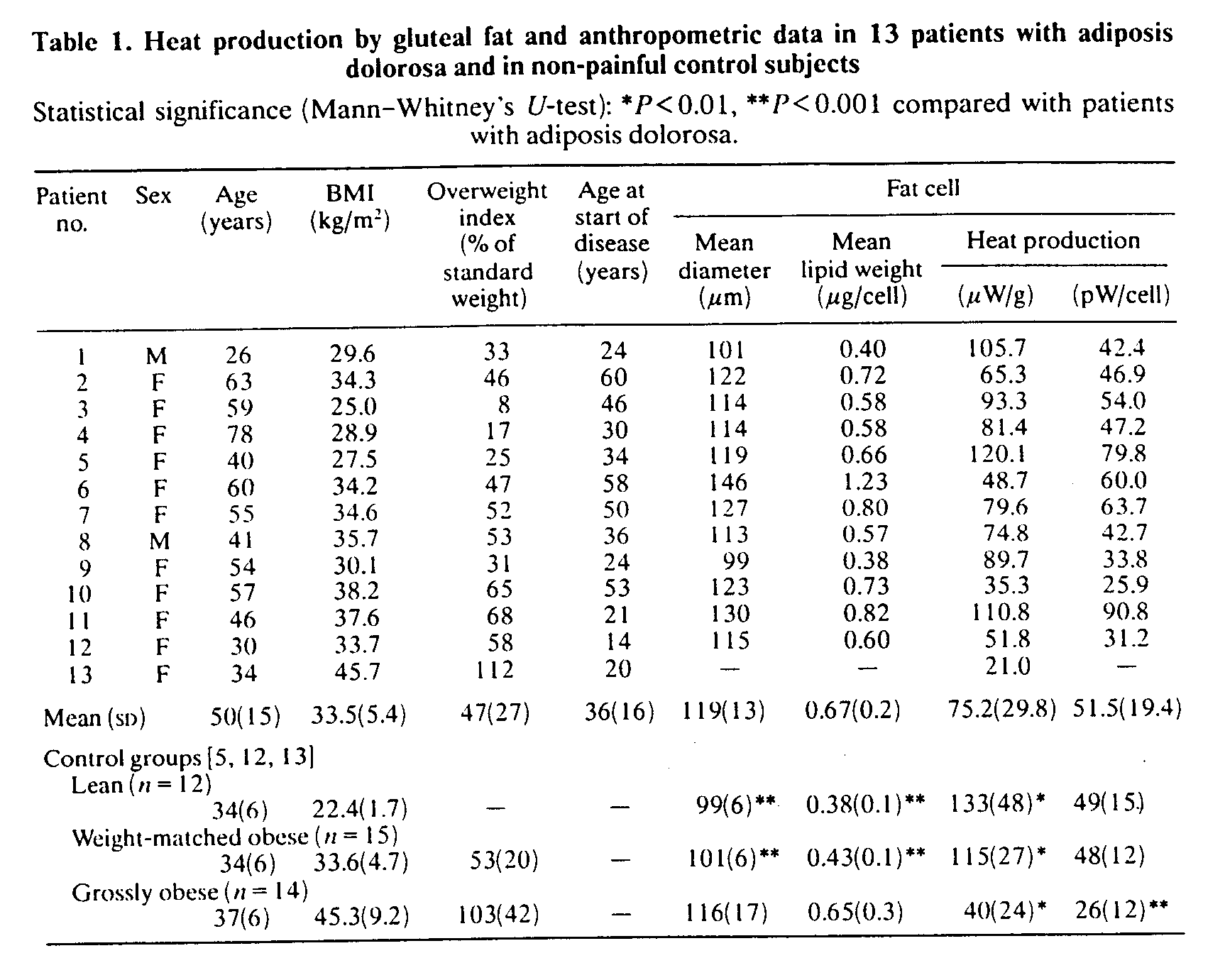
Fat-cell heat production (Table 1)
The mean heat production rate, 75 mW/g of adipocyte lipid weight (SD 30), was significantly lower (P< 0.01) than the corresponding values of both lean healthy normal subjects and weight-matched obese control subjects. However, mean fat-cell weight as well as adipocyte diameter, 118.5/mm (SD 12.8), were significantly greater (P < 0.001 ) than in these two groups. Thus when the heat production rate was expressed in pW/cell there were no significant inter-group differences. On the other hand, when compared with the group of grossly obese subjects, the patients with adiposis dolorosa had significantly higher heat production in mW/g as well as in pW/cell (P<0.01 and P< 0.001, respectively). Heat production, in mW/g or pW/cell, showed no correlation with patient age in either study group (rs=-0.1 and 0.2, respectively) or in the three control groups (rs= -0.1 to 0.4); patients younger than 50 years of age (n = 6) had a heat production of 57.4 pW/cell (SD 26.2), which did not significantly differ from the values of the lean and weight-matched groups.
When expressed per g of fat tissue, heat production was inversely correlated with BMI in the study group (rs=-0.61, P<0.03, Fig. 1) as well as in the weight-matched control group (rs=-0.58, P<0.04). No such correlations were found in the two other control groups (rs = - 0.3 to - 0.1 ). Cell diameter was significantly correlated with the total adipose tissue (rs = 0.70, P<0.02), but it did not correlate significantly with BMI (rs = 0.5) in the study group. Heat production in mW/g was not significantly correlated with cell size (rs = -0.3), and no significant relationships were found between the value in pW/cell and either BMI (rs = -0.1) or cell size (rs = 0.50). In the weight-matched control group, heat production in gW/g, but not in pW/cell, was inversely correlated with cell size (rs = - 0.58, P< 0.03).
D-Thyroxine administration, which gave no symptomatic relief, decreased the heat production rate to 61 mW/g (SD 35) or 43 pW/cell (SD 25) from 81 mW/g (SD 26) or 59 pW/cell (SD 29) before therapy. Activity of adipose tissue LPL increased to a median of 15.3 m-units/g of lipid from 4.5 m-units/g of lipid. All these changes were, however, non-significant (Wilcoxon's test). After treatment, mean fat-cell weight increased slightly to 0.76 mg (SD 0.24), whereas body weight was unchanged. Fatty acid composition was almost identical in three patients who had another biopsy performed after treatment.
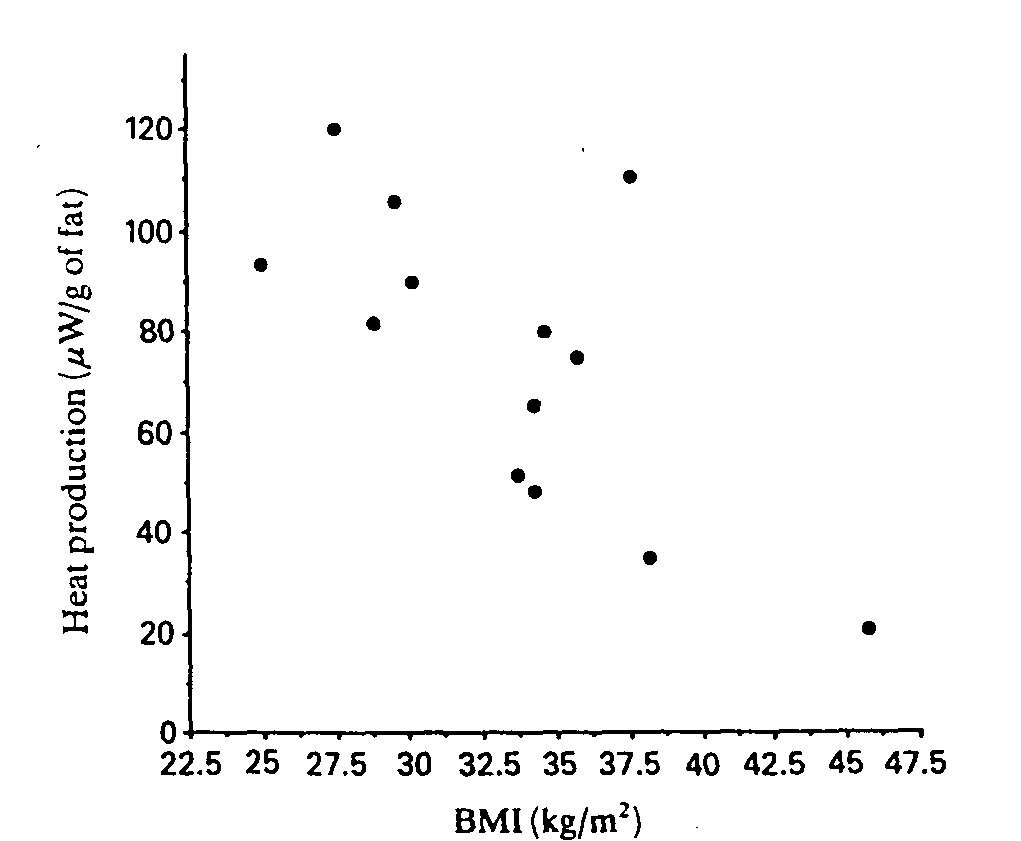
Fig. 1. Heat production by gluteal fat in 13 patients with adiposis doiorosa plotted against BMI. rs=-0.61, P<0.03.
The woman with unilateral pain was biopsied on both the painful and the unaffected buttock and the following values were found: heat production rate, 19.4 and 97.7 mW/g, or, if expressed in pW/cell, 8.2 and 37.2, respectively; mean cell diameter, 102.6 and 97.0 /mm, respectively. Fatty acid composition was almost identical on the two sides and LPL activity was low, 3.2 and 2.5 m-units/g of lipid, respectively.
Adipose tissue fatty acid composition (Table 2), plasma lipoproteins and LPL activity
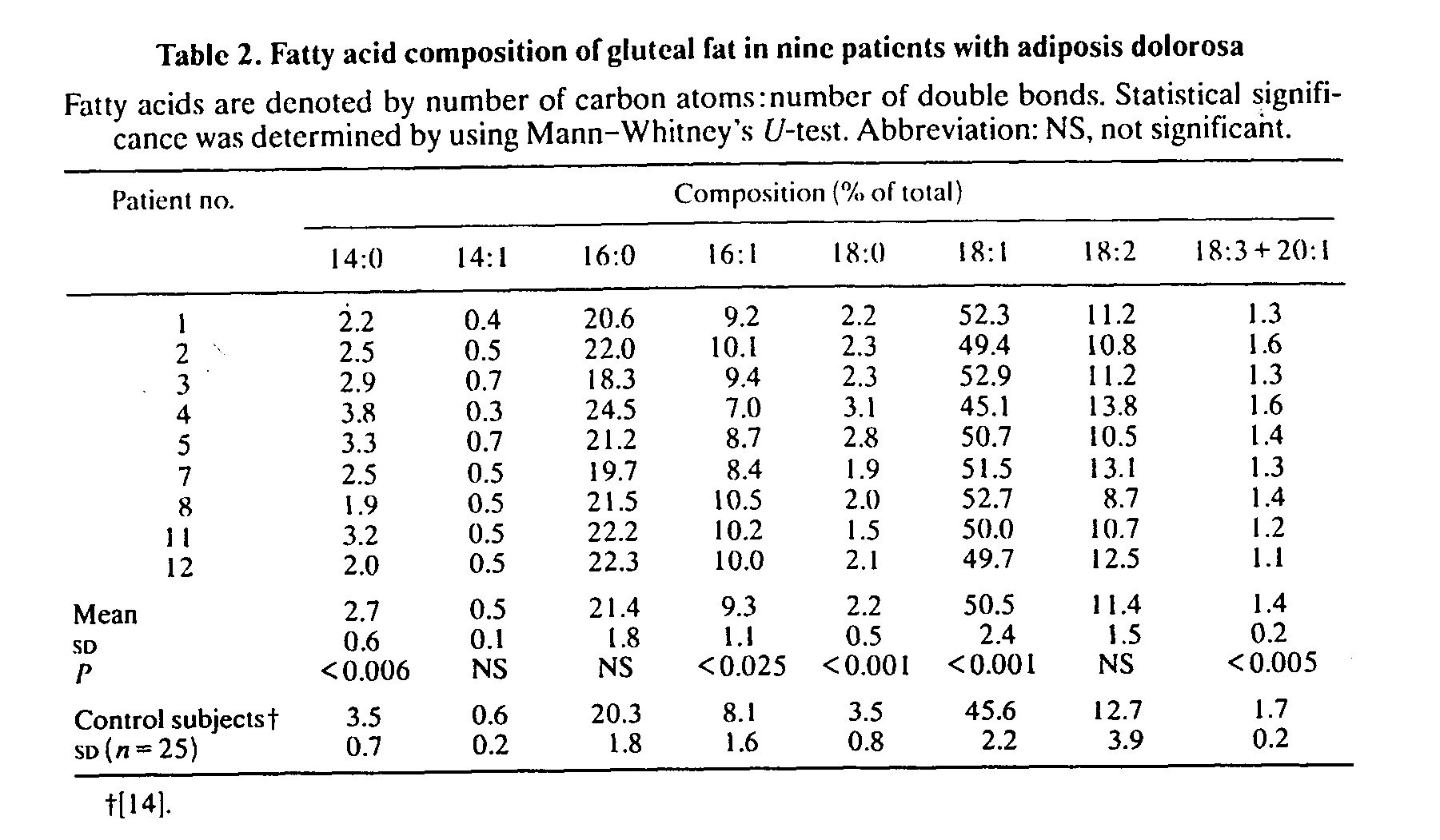
Patients had significantly decreased proportions of 14:0, 18:0 and 18:3 + 20:1 (fatty acids denoted as number of carbon atoms:number of double bonds), whereas the relative amounts of 16:1 and 18:1 were significantly increased compared with samples from healthy control subjects. The statistical differences persisted even when a slight influence of sex on fatty acid composition {14] was taken into consideration. The relative distribution of total saturated acids, 26.7% (SD 2.3), was not different from that of the controls, 27.3% (SD 2.4), whereas the proportion of total monounsaturated acids, 60.5% (SD 3.3), was significantly higher (P< 0.002) than that of the control subjects, 55.2%. The proportion of stearic acid (18:0) was significantly correlated with the overweight index (r=-0.8, P<0.02, Fig. 2), an observation also made in the lean control group (L. Jacobsson, F. Lindgärde, R. Manthorpe & B. Äkesson, unpublished work). No relation was found between either fat-cell heat production or cell size and fatty acid composition.
Four patients had hypercholesterolaemia with plasma lipid concentrations of >7 mmol/l. The mean plasma levels were: cholesterol, 6.3 mmol/l (SD 1.5); LDL cholesterol, 4.3 mmol/l (SD 1.3); HDL cholesterol, 1.06 mmol/l (SD 0.28); TAG, 1.7 mmol/l (SD 1.2). Adipose tissue LPL activity, as analysed in seven of the patients, had a median value of 4.7 m-units/g of lipid wet weight (interquartile range 3.9-8.0 m-units/g of lipid wet weight), which is slightly, although not significantly, lower than our reference value, 5.2-14.8 m-units/g of lipid wet weight.
DISCUSSION
Intractable painful obesity is a characteristic feature of adiposis dolorosa [1-4] and our patients fulfilled this criterion. Even if the patients appeared to have rather stable body weights at the time of biopsy, the natural course of the disease, at least during the first decade, included a steady weight gain and increasing pain. They were almost 50% overweight as compared with our reference subjects.
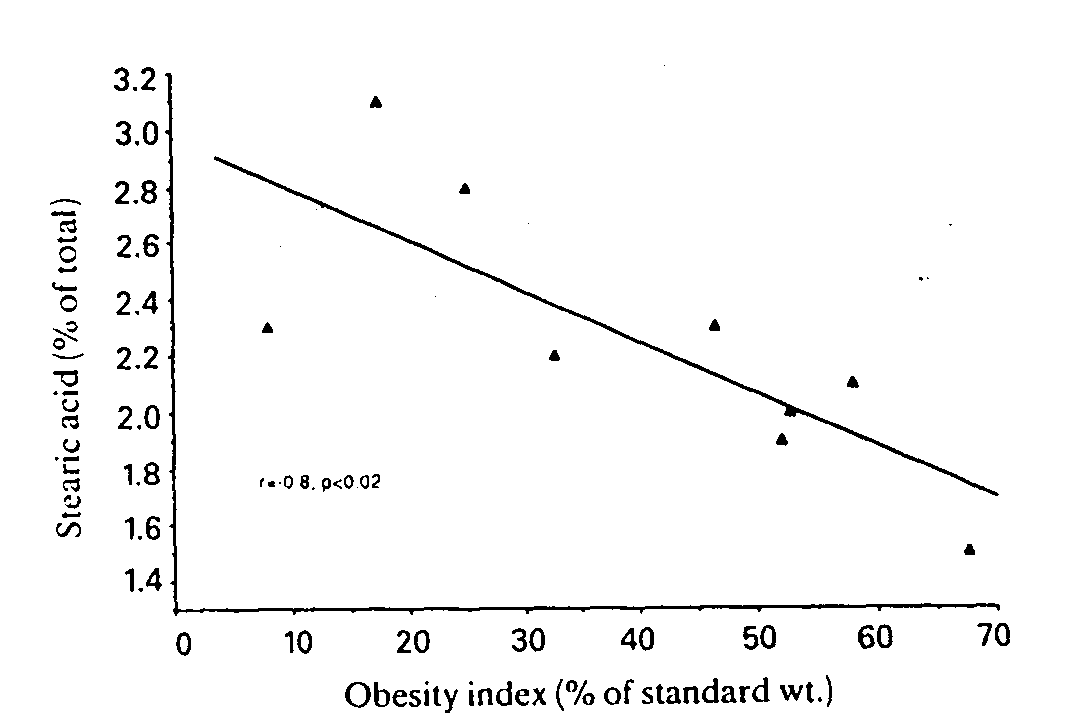
Fig. 2. Plot of the proportion of stearic acid (18:0) in adipose tissue against obesity index for patients with adiposis dolorosa. Linear regression equation: y= -0.018x+ 3. r= -0.8, P<0.02.
As the subcutaneous tenderness was diffuse and was not confined to 'lipomas', we chose to take the biopsies from the same painful area in all subjects, namely the buttocks, which had little or no granularity. Fat cells were larger than those of weight-matched subjects and similar in size to those of a grossly obese group. Heat production, as expressed per cell, was, however, almost twice as high as in the latter group. The pathophysiological significance of this is unclear, but it may possibly distinguish adiposis dolorosa from ordinary non-painful obesity. One could speculate if the difference in heat production compared with the grossly obese group might be dependent on a higher sympathetic activity due to nociceptive stimuli in the painful tissue. There is in fact one case report [19] suggesting sympathetic dysfunction in Dercum's disease, but there are also indications of reduced sensitivity of the painful tissue to noradrenaline [20]. Generally, however, the influence of the sympathetic nervous system on adipose tissue as well as on the control of fat-cell size are still poorly understood [21].
We did not find any significant association between heat production and cell size in the study group, but this was the case in the weight-matched control group and has been reported previously in normal rats [22]. In our previous investigations it was further noted that after weight reduction, due to either surgical gastroplasty [12} or dietary restriction [13], heat production, notably that expressed per g of adipose tissue, increased from initially subnormal values as cell size decreased. Whether our patient with the remarkable unilateral pain should be classified as having Dercum's disease or not is an open question. Nevertheless, she displayed clear side differences in cellular heat production, with markedly lower values and somewhat larger fat cells on the painful side.
Our present findings merit further metabolic study, especially in the light of a previous report on 11 patients with adiposis dolorosa [19] showing an inhibited lipolysis, reduced glucose utilization (cf. [23]) and decreased insulin sensitivity in painful lipomas compared with non-painful fat. A hypothesis was also presented by Blomstrand et al. [4] on possible defects in long-chain monounsaturated fatty acid biosynthesis emanating from an examination of 'lipomas' in two patients who exhibited a more localized form of disease with painful fat deposits confined to the legs. This defective synthesis was, however, not associated with an abnormal adipose tissue fatty acid composition, which actually argues against their proposed mechanism. Contrary to this, we found an increased, not a decreased, proportion of, for example, oleic acid (18:1) and a significantly increased relative percentage of total monounsatu- rated acids as compared with healthy control subjects. One could ask if this was due to the different dietary pattern of our patients? However, the proportion of the essential linoleic acid (18:2) that can be assumed to have originated only from the diet did not differ between patients and control subjects. No major abnormality in adipose tissue fatty acid composition has otherwise been reported in ordinarily obese female subjects [24]. The inverse relationship that we found between the proportion of stearic acid (18:0) and the obesity index gave no hint of a biochemical abnormality as it was similar to that observed in the healthy subjects (cf. [25]).
So far, our study has been confined to the adipose tissue of patients with adiposis dolorosa and the data do not explain the nature of the diffuse chronic pain. It is concluded that the fat cells from these patients were markedly enlarged and that cellular heat production was higher than in grossly obese subjects who had fat cells of similar size. The metabolic rate was, however, not different when compared with normal-sized fat cells from lean healthy or from weight-matched obese non-painful subjects. The pathophysiological importance of an altered fatty acid composition, with increased monounsaturated acids, in the painful adipose tissue remains to be determined. A metabolic pathogenetic factor cannot be ruled out in adiposis dolorosa, and further study is needed for example of the mechanisms of the control of adipocyte size.
ACKNOWLEDGMENTS
We thank Ms Lilly Berlin, Gerd Nilsson and Mrs Berit Persson for excellent technical assistance. D-Thyroxine was supplied by Boehringer Mannheim, Germany. The study was supported, in part, by grants from the Malmöhus County Council and Påhlsson's Foundation, Malmö, Sweden.
REFERENCES
1.
Boiler, R. Die Novocainbehandlung des Morbus Dercum. Klin Wochenschr. 1934; 50, 1786-9.
2.
Iwane, T., Maruyama, M., Matsuki, M., Ito, Y. & Shimoji, K. Management of intractable pain in adiposis dolorosa with intravenous administration of lidocaine. Curr. Res. Anesth. Analg. 1976; 55, 257-9.
3.
Atkinson, R.L. Intravenous lidocaine for the treatment of intractable pain of adiposis dolorosa. Int. J. Obes. 1982; 6, 351-7.
4.
Blomstrand, R., Juhlin, L., Nordensram, H., OIsson, R., Werner, B. & Engström, J. Adiposis dolorosa associated with defects of lipid metabolism. Acta Derm. Venereol. 1971; 51, 243-50.
5.
Monti, M., Nilsson-Ehle, p., Sörbris, R. & Wadsö, I. Micro-calorimetric measurement of heat production in isolated human adipocytes. Scand. J. Clin. Lab. Invest. 1980; 40, 581-7.
6.
Lundh, Bj. Variation of body weight with age, sex and height. An index for classification of obesity. Acta Med. Scand. 1985; 218, 493-8.
7.
Sjöström, L. Comments with respect to Dr Wolfgang Leonhardt's letter. Int. J. Obes. 1989; 13, 378-80.
8.
Ljung, O. Adipositas dolorosa (Dercum's sjukdom). Forsk. Prak. (Sandoz) 1978; 10, 63-5.
9.
Smith, U., Sjöström, L. & Björntorp, P. Comparison of two methods for determining human adipose cell size. J. Lipid Res. 1972; 13, 822-4.
I0.
Hirsch, J. & Gallian, E. Methods for the determination of adipose cell size in man and animals. J. Lipid Res. 1968; 9, 110-9.
11.
Wadsö, I. Calorimetric techniques. In: James, A.M., ed. Thermal and energetic studies of cellular biological systems. Bristol: Wright, 1987: 34-67.
12.
Olsson, S.-Å., Monti, M., Sörbris, R. & Nilsson-Ehle, P. Adipocyte heat production before and after weight reduction by gastroplasty. Int. J. Obes. 1986; 10, 99-105.
13.
Sörbris, R., Monti, M., Nilsson-Ehle, P. & Wads& I. Heat production by adipocytes from obese subjects before and after weight reduction. Metab. Clin. Exp. 1982; 31, 973-8.
14.
Jacobsson, L., Lindgärde, F., Manthorpe, R. & Åkesson. B. Correlation of fatty acid composition of adipose tissue lipids and serum phosphatidylcholine and serum concentrations of micronutrients with disease duration in rheumatoid arthritis. Ann. Rheum. Dis. 1990; 49, 901-5.
15.
Beynen, A.C. & Katan, M.B. Rapid sampling and long-term storage of subcutaneous adipose tissue biopsies for determination of fatty acid composition. Am. J. Clin. Nutr. 1985; 42, 317-22.
16.
Nilsson-Ehle, P. & Ekman, R. Rapid, simple and specific assays for lipoprotein lipase and hepatic lipase. Artery 1977; 3, 194-209.
17.
Nilsson-Ehle, P. Human lipoprotein lipase: comparison of assay methods. Clin. Chim. Acta 1974; 54, 283-91.
18.
Friedewald, W.T., Levy, R.l. & Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol without use of the preparative ultracentrifuge. Clin. Chem. 1972; 18, 499-506.
19.
Skagen, K., Petersen, P., Kastrup, J. & Nørgaard, T. The regulation of subcutaneous blood flow in patients with Dercum's disease. Acta Derm. Venereol. 1986; 66, 337-9.
20.
Leites, S.M., Davtyan, N.K. & Emanuel, V. Ya. Pathophysiological characteristics of adipose tissue in Dercum's syndrome [In Russian]. Patol. Fiziol. Eksp. Ter. 1972; 16, 47-51.
21.
Dalziel, K. The nervous system and adipose tissue. Clin. Dermatol 1989; 7, 62-77.
22.
Nilsson-Ehle, P. & Nordin, G. Microcalorimetric studies on the total metabolic activity of fat cells. Int. J. Obes. 1985; 9 (Suppl. 1), 169-72.
23.
Taniguchi, A., Okuda, H., Mishima, Y. et al. A case of adiposis dolorosa: lipid metabolism and hormone secretion. Int. J. Obes. 1986; 10, 277-81.
24.
Heifernan, A.G.A. Fatty acid composition of adipose tissue in normal and abnormal subjects. Am. J. Clin. Nutr. 1964; 15, 5-10.
25.
Insull, W., Jr. & Bartsch, G.E. Fatty acid composition of human adipose tissue related to age, sex, and race. Am. J. Clin. Nutr. 1967; 20, 13-23.
© 1991, Clinical Science. Reprinted here with the kind permission of the author, Dr. Birgher Fagher.
Return to the List of Articles
Return to the
Dercum's Disease Home Page
Last updated 18 Nov 2003
Comments about the web page format
should be sent to the Don
Please don't forget that the information provided on this site is designed to support, not replace, the relationship that exists between a patient and his or her physician.