Early report
Volume 347, Number 9018 ![]() 29 June 1996
29 June 1996
Early report |
Volume 347, Number 9018 |
| Lethal infection by a previously unrecognised metazoan parasite | ||
Mónica Santamarķa-Frķes, Luis Felipe Fajardo L-G, Mitchell L Sogin, Peter D Olson, David A Relman
Department of Pathology, Kaiser-Permanente Medical Center, Santa Clara, CA, USA (M Santamarķa-Frķes MD); Department of Pathology, Stanford University School of Medicine, Stanford, CA and Veterans Affairs Palo Alto Health Care System, Palo Alto, CA (Prof L F Fajardo L-G MD); Center for Molecular Evolution, Marine Biological Laboratory, Woods Hole, MA (M L Sogin PhD); Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, CT (P D Olson MS); and Departments of Medicine, Microbiology and Immunology, Stanford University School of Medicine, Stanford, CA; and Veterans Affairs Palo Alto Health Care System, Palo Alto, CA, USA (D A Relman MD)
Correspondence to: Prof Luis F Fajardo L-G, Stanford University, Department of
Pathology (113), VA Palo Alto Health Care System, 3801 Miranda Avenue, Palo Alto, CA
94304, USA
![]() Summary
Summary
![]() Introduction
Introduction
![]() Patient and methods
Patient and methods
![]() Results
Results
![]() Discussion
Discussion
![]() References
References
Background New microbial pathogens or variant clinical manifestations of known organisms may be first found in immunodeficient patients. An HIV-infected man developed a rapidly-enlarging abdominal mass, suggestive of a neoplasm, that subsequently invaded his liver and caused death. Initial studies showed unusual tissue morphology that could not be matched with any known disease process.
Methods Tissues obtained from biopsy at laparotomy and necropsy were studied by light microscopy, immunohistochemistry, electron microscopy, and broad-range ribosomal DNA-amplification and sequence analysis.
Findings Tissue lesions were characterised by peculiar cytoplasmic sacs containing minute cells with very prominent nucleoli. The pathological process was recognised as a parasitic infection, although its features were different from those of any known eukaryotic pathogen. Phylogenetic analysis of a 357 bp 18S rDNA sequence amplified directly from the involved tissue indicated that the causative agent was a previously-uncharacterised cestode.
Interpretation Fatal disease produced by this newly recognised cestode may not be limited to immunodeficient hosts. Awareness of this metazoan infection may allow early diagnosis--by morphology and DNA sequence analysis--and perhaps successful treatment of subsequent cases.
HIV infection and AIDS have drawn attention to a number of previously unknown
opportunistic infections. Some of the infectious agents have resisted classification or
propagation in the laboratory. Parasitic infections contribute to the morbidity and
mortality associated with HIV infection1,2 and atypical parasite behaviour and
clinical presentations have been associated with immunosuppression.3,4 We
encountered a micro-organism that caused a fatal illness in a patient with AIDS. This
organism resisted identification until analysis of a small subunit ribosomal DNA (ss rDNA)
sequence from infected tissue, indicated that the pathogen was a
previously-uncharacterised plathyhelminth, probably a cestode.
![]()
A 44-year-old man with HIV infection for 5 years and a recent diagnosis of AIDS (CD4+ cell count: <100310-4/L) was admitted to hospital in March, 1994, with a 2-month history of abdominal and low-back pain, weight loss, night sweats, and fever. During the previous 5 years, he had several upper-respiratory tract infections and episodes of diarrhoea but no specific opportunistic infection. Stool examination on two occasions was normal. Current medications were zidovudine, dapsone, and pentamidine. He had a previous history of hepatitis B infection and treated syphilis. He had been in a monogamous homosexual relationship for 18 years. He worked as an accountant, lived in the San Francisco Bay Area, and had never travelled outside the USA. He went camping in the area in which he lived. He had two dogs with which he had close contact.
On admission he was febrile (39°C). His abdomen was soft and non-tender; a periumbilical 8310 cm mass was palpable. An abdominal computed tomogram (CT) scan showed para-aortic, paracaval, and mesenteric nonhomogeneous lobulated masses, up to 9 cm long in their greatest dimension. A CT-directed biopsy yielded no diagnostic material. Colonoscopy and biopsies of rectum and terminal ileum were normal. The patient had an exploratory laparotomy and biopsy of the mesenteric mass 1 week after admission.
Because he was presumed to have a protozoan infection, metronidazole was started, but discontinued after 7 days due to intolerance. Severe abdominal pain persisted. He developed anaemia (Hb 9·5 g/dL) and intractable nausea and vomiting with dehydration. He progressively deteriorated, and died 9 weeks after the laparotomy.
Morphology Light microscopy and immunohistochemistry of paraffin-embedded samples from biopsy and necropsy material were done, and a biopsy sample was examined by transmission electron microscopy.
Ribosomal DNA sequence analysis Fresh-frozen liver tissue was divided into
abnormal and normal portions by gross inspection. Segments of these two types of tissue
measuring 3 mm3 were digested and processed in parallel for PCR analysis.5
Broad-range eukaryotic ss rDNA amplification reactions included 20 pmol of primers 1 FPL
(Eukarya-specific,5'GCGGATCCGCGGCCGCTGGTTGATCCTGCCAGT 3') and 1520RPL (universal,
5'GCGGATCCGCGGCCGCYGCAGGTTCACCTAC 3'),6 200 nmol MgCl2, 2·5 U Taq
polymerase, and 1-10 µL (1-10%) of the tissue digests in a total volume of 100 µL. With
the hot-start technique, 40 PCR cycles were done with an annealing temperature of 60°C.
PCR products were purified and sequenced directly with the Dye Deoxy Terminator Cycle
Sequencing Kit (Perkin-Elmer, Foster, City, CA, USA). The ss rDNA sequence obtained in
this study was aligned against a database of 80 eukaryotic ss rDNA sequences according to
conserved primary and secondary structures. Phylogenetic analyses were based upon
comparisons of 303 sites that were judged by these criteria to be in alignment.
Phylogenetic trees were constructed with two algorithms: maximum parsimony using the
software package PAUP3.1.1;7 and maximum-likelihood.8 Bootstrap
values for the dendrogram generated by PAUP were obtained from 100 resamplings.
Distance-matrix analysis followed the least squares method.9
![]()
The open-biopsy material of the mesenteric mass consisted of dense fibroadipose tissue within a rim of lymphatic tissue with an extensive inflammatory reaction characterised by fibrinous exudate, macrophages, and scattered groups of neutrophils forming microabscesses. There were no granulomas or eosinophils.
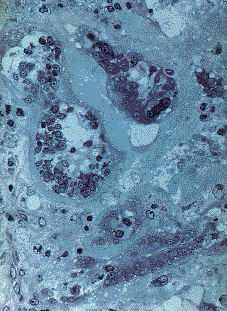
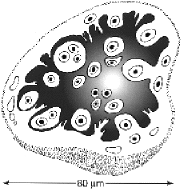
Randomly dispersed in this stroma were numerous nests, or sacs, of the microorganism (figures 1 and 2). The sacs measured 85 µm in average diameter, were oval or flask-shaped, and composed of an outer amphophilic shell (wall) that stained weakly with hematoxylin and eosin, and a central cavity containing characteristic cells. These cells had a mean diameter of 6·7 µm; their cytoplasm was dense and their most prominent feature was a small spherical nucleus (2 to 4 µm; clearly smaller than most human nuclei) with a very large round nucleolus. Some parasitic cells were multinucleated. A few sacs had ruptured and their cells were loose in the surrounding stroma. Although invasion of the sacs by histiocytes or neutrophils was seen, phagocytosis was not.
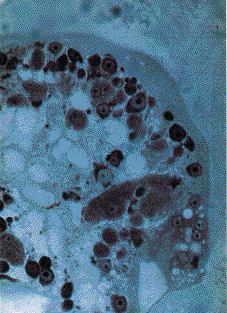
The possible human origin of these cells was explored with immunohistochemical reagents for normal or neoplastic human antigens, including cytokeratins, human chorionic gonadotropin, alpha fetoprotein, epithelial membrane antigen, S-100 protein, vimentin, desmin, and multiple lymphoid markers. All of these markers were negative. The cells did not react with the usual stains for bacteria (such as Gram) or fungi (silver methenamine, Gridley etc). They were acid-fast negative (Ziehl-Neelsen) and did not contain acid mucopolysaccharides (with Alcian blue at pH 1·5). Although the cells were periodic acid-Schiff (PAS)-negative there was abundant PAS-positive and diastase-sensitive amorphous material, presumably containing glycogen, among the cells.
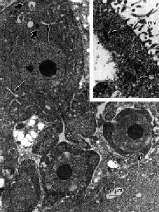
Electron microscopy of the biopsy sample showed that the wall of the sacs was composed of cytoplasmic material containing some glycogen and a few nuclei (figure 3). The outer surface of the wall was studded with a continuous layer of microvilli, while the inner surface projected lamellipodia toward the lumen. Inside the sacs were the minute cells seen by light microscopy. Those cells in the periphery, between lamellipodia, seemed to originate from the wall and often were continuous with it. They had loosely distributed, small mitochondria and were larger than the cells closer to the centre of the sacs. Those cells in the center had denser cytoplasm, suggesting a process of maturation and/or senescence from the periphery to the centre of the sacs. All cells lacked rough endoplasmic reticulum, phagolysosomes, and junctional complexes (figure 4). Neither cell wall nor chloroplast were seen. Although these features suggested a metazoan parasite, there were no structures such as eggs, tegument, scoleces, or cilia.
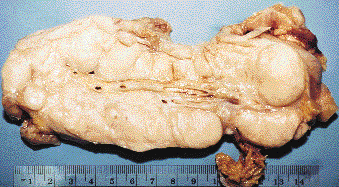
Necropsy less than 6 h after death showed large mesenteric and retroperitoneal, lobulated tan masses, up to 20 cm in greatest dimension (figure 5). Smaller nodules were present in the diaphragm and lower retrosternal soft tissue. The liver, which was markedly enlarged, had bulky, irregular tan lesions that involved approximately 75% of the parenchyma. The abdominal masses and the liver had extensive necrosis and contained numerous plastic sacs, most of which were ruptured. Rare cytomegalovirus inclusions were observed in human cells within the mesenteric mass. There was severe bronchopneumonia. No evidence of cancer was found. Cultures from blood, liver, and mesenteric masses were negative for bacteria and fungi, except for light growth of Candida glabrata from one of the mesenteric samples.
A PCR product of approximately 400 bp was consistently detected in digests of diseased
liver tissue with reactions using broad range Eukarya ss rDNA primers. No other PCR
products were detected. On the other hand, digests of normal portions of liver from the
same patient only yielded an approximately 1·8 kb PCR product (data not shown). A 1·8 kb
DNA band would have been expected from amplification of human 18S rDNA with these primers.
The disease-associated PCR product contained 357 bp, excluding the two PCR primers. This
357 bp sequence corresponded to the ss rDNA of a previously-uncharacterised metazoan
(GenBank accession number
U 58674). At approximate position 360 within the ss rDNA of the most closely-related
organisms, there is a region in which 14 out of 15 nucleotides in a reverse complementary
orientation match the 3' end of primer 1520 RPL, suggesting that 1520 RPL mispriming led
to the amplification of this unexpectedly small DNA fragment. Parsimony, distance, and
neighbour-joining phylogenetic analyses indicated that the case parasite is most
closely-related to the cestodes (figure 6); bootstrap analysis strongly supported this
association.
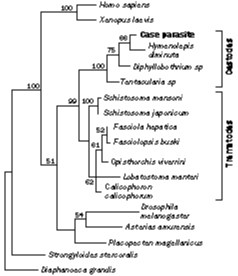
These results and the morphological data, suggest that the nests of cells represent a
tissue-invasive larval stage of a cestode-like organism. Given the relatively few cestode
ss rDNA sequences that were available for our analysis and the paucity of sequence data
from the case parasite, we could not determine precise evolutionary relationships between
this parasite and other cestodes.
![]()
This patient had a progressive, tissue-destructive parasitic infection. Despite rigorous histological examination, the nature of the pathogen could not initially be identified. Efforts to detect signature elements and compounds in the diseased tissues by energy-dispersive and lipid-profile analysis (data not presented) failed to provide specific clues. Small subunit rDNA sequences allow the inference of phylogenetic relationships without reliance on cultivation or growth-based phenotypes. For this reason we amplified these sequences directly from infected tissue. Although there are very few cestode ss rDNA sequences available, our analysis suggests that this parasite is a previously-unknown cestode.
The lack of sequence similarity of this parasite with previously-characterised organisms is consistent with its unique histologic features. We have found no reports of a human-associated parasite morphology or clinical course that resemble those described in this case. In fact neither we, nor any of the experts on human and veterinary parasitic diseases we consulted, have seen this micro-organism before. There is a vague similarity between this case and one reported by Connor et al in 1976 and interpreted as a possible embryonal stage of aberrant spirometra (sparganum).3 In one of their micrographs the minute cells and the enveloping sac slightly resemble our parasitic nests; other aspects, including the ultrastucture, are rather different. The morphology of this parasite suggests that it was in the larval stage.3,11 No evidence of maturation (no change in morphology) occurred in the 3-months between biopsy and death.
Assuming that this is a newly recognised human parasite infection, we could only speculate about the possible route of entry and its development within the host. Since the initially detected lesion was in the mesentery, infection through the mouth by eggs or larvae may have occurred, with subsequent penetration through the intestinal wall and dissemination into lymph nodes. The infection may have occurred either from ingestion of contaminated meats, fish, crustaceans, etc, containing procercoid larvae (as in the case of sparganosis) or ingestion of eggs or proglottids as in the case of taeniasis.12
As for the source, we have considered the possibility that the dogs with which the patient was particularly affectionate (to the point of biting their paws and noses) may have harboured the organism. Although no parasites were found in the dogs faeces, this source cannot be ruled out. The sexual partner of this patient is apparently healthy.
From studies in mice it appears that helper T lymphocytes (especially the Th1 subtype) are important in the protective immunity against the cestode Hymenolepis nana.10 Thus, by analogy, this immunodeficient patient might have lacked selective protection against cestode infections.
Awareness of these unusual clinical and pathological features may allow early diagnosis and treatment in subsequent cases. Additional sequence-based studies may be necessary for the complete characterisation of this metazoan.
The authors greatly appreciate the expert opinions of the following persons: Jon Kosek, Michael Hendrickson, Tracy Tingle, Paul Basch, and Monte Laskosky (Stanford, CA); Daniel Connor (Washington, DC); Jack Frenkel (Santa Fe, NM); Daniel Gould (Fort Collins, CO); Eileen Johnson (Davis, CA); Govida S Visvesvara (Atlanta, GA) and David C White (Oak Ridge, TN). Helen Kwan, Kristine Yoder, Chris Morrow, and Donna Buckley provided technical assistance.
Supported in part by Veterans Affairs Research Fund FAJ004 (LFF), Lucille P Markey
Charitable Trust (DAR), NIH Grant GM32964 (MLS), NSF (BSR-91-96213) (PDO), and University
of Connecticut Research Foundation Grant awarded to C S Simon (PDO). DAR is a Lucille P
Markey Biomedical Scholar.
![]()
1. Gutierrez Y. Diagnostic pathology of parasitic infections with clinical correlations. Philadelphia: Lea & Febiger, 1990.
2. Wittner M, Tanowitz HB, Weiss LM. Parasitic infections in AIDS patients. Cryptosporidiasis, isosporiasis, microsporidosis, cyclosporiasis. Inf Dis Clin North Am 1993; 7: 569-86.
3. Connor DH, Sparks AK, Strano AJ, Neafie RC, Juvelier B. Disseminated parasitosis in an immunosuppressed patient. Possibly a mutated sparganum. Arch Pathol Lab Med 1976; 100: 65-68.
4. Zumla A, Croft SL. Chemotherapy and immunity in opportunistic parasitic infections in AIDS. Parasitology 1992; 105: S93-101.
5. Relman DA, Schmidt TM, MacDermott RP, Falkow S. Identification of the uncultured bacillus of Whipple's disease. N Engl J Med 1992; 327: 293-301.
6. Medlin L, Elwood HJ, Stickel S, Sogin ML. The characterization of enzymatically amplified eukaryotic 16S-like rRNA coding regions. Gene 1988; 71: 491-99.
7. Swofford DL. PAUP: Phylogenetic analysis using parsimony, version 3.0s,. Champaign, IL: Illinois Natural History Survey, 1991.
8. Olsen GJ, Matsuda H, Hagstrom R, Overbeek R. fastDNAmL: a tool for construction of phylogenetic trees of DNA sequences using maximum likelihood. Comput Appl Biosci 1994; 10: 41-48.
9. DeSoete G. A least squares algorithm for fitting additive trees to proximity data. Psychometrika 1983; 48: 621-26.
10. Asano K, Okamoto K. Murine T-cell clones specific for Hymenolepis nana: generation and functional analysis in vivo and in vitro. Int J Parasitol 1991; 21: 891-96.
11. Berrada-Rkhami O, Gabrion C. The fine structure of the embryonic envelopes before and after hatching in bothriocephalids: physiological and ecological significance. Parasitol Res 1990; 76: 251-62.
12. Orihel TC, Ash LR. Parasites in human tissues. American Society of Clinical Pathologists. Chicago, IL, 1995.
Return to the List of Articles
Return to the
Dercum's Disease Home Page
Last updated 18 Nov 2003
Comments about the web page format
should be sent to the Don
Please don't forget that the information provided on this site is designed to support, not replace, the relationship that exists between a patient and his or her physician.